Quality & Compliance Services
bioMérieux ist Ihr Partner zu Fragen der Risikobewertung, Qualitätssicherung und Compliance
- Competent, individual validation concepts
- Extensive product range for immunoassays for quality assurance (myQC platform for VIDAS systems)
Benötigen Sie weitere Informationen?
The regulatory environment is becoming increasingly stringent as we strive to continually improve patient care while facing ever-increasing workloads and constant cost pressures. Ensuring quality and compliance management is now more important than ever. Working with bioMérieux on quality and compliance management gives you the confidence to know that everything is under control. Be prepared for the future .
Validation packages
Accreditation requirements include validating your systems and methods – increasingly complex and time-consuming procedures . To help you through the “labyrinth” of validations and save valuable time, we provide you with an individualized validation package that allows you to quickly and correctly validate your bioMérieux systems step by step. We support your laboratory with:
- Validation documents : We supply the documents for the entire validation process and provide additional required documents on a sustained basis. This supports the qualification of systems, the validation of reagents before they are used in routine use and the verification of methods under application conditions.
- Training your employees on system validation : Together with a proven bioMérieux expert, you will set the validation process in motion. The training courses tailored to your needs include demonstrations of the correct use of the devices, user manuals, method validation software and answers to the most important questions about compliance and quality.
Risk analysis procedures e
We have developed guidelines tailored to your needs for implementing risk analysis in accordance with ISO 15189 regulations.
Quality control monitoring
Our “Performance Solutions” offer comprehensive services – myQC solutions (Internal Quality Control (IQC)) – for immunoassays (VIDAS ® systems) that support you in complying with ISO & CAP standards. Fast and successful internal quality control (IQC) ensures the quality of the results by detecting drifts at an early stage and evaluates results by comparing data with other laboratories.
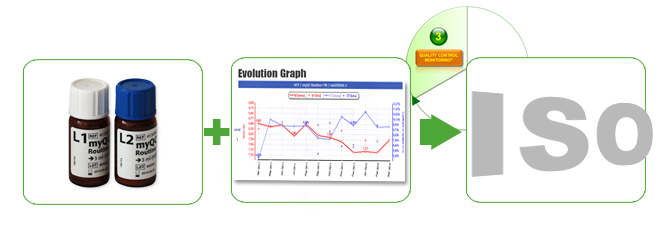
- Materials (vials) for quality control (QC) : 3 versions are available for the IQC of the VIDAS ® systems:
- QC ROUTINE + for the most important parameters of immunodiagnosis (VIDAS ® Thyroid, VIDAS ® Tumor Markers, VIDAS ® Fertility,…)
- QC CARDIAC with relevant markers (VIDAS D-Dimer II, VIDAS ® NT Pro BNP, VIDAS ® Troponin I, VIDAS ® Myoglobin)
- QC SPECIALTY for VIDAS ® BRAHMS PCT
- Web application for exchanging opinions about QC : An application will soon be available for VIDAS ® customers (VIDAS ® , mini VIDAS ® and VIDAS ® 3) with which you can compare your internal quality assurance with those of other laboratories worldwide. Connection of devices via VILINK ™ software.
- New service offerings for accreditation & QC analysis : In addition to our regular customer service, we offer you the option of telephone and on-site service if required.
myQC for VIDAS ®
In order to enable our customers to monitor the performance data of their VIDAS systems, they have the opportunity to exchange information and experiences with other VIDAS users worldwide via the myQC platform.





